Mingli Liu, MD, PhD, MSCR
Associate Professor
Microbiology, Biochemistry & Immunology
Location: Medical Education Building | MEB C-316
Phone: (404) 752-1850
E-mail: mliu@msm.edu
Education
GRADUATE
Peking Union Medical College & Tokyo University
Degree: Doctor of Philosophy in Hematology/Oncology
People’s Liberation Army Postgraduate Medical School
Degree: Master of Science in Hematology/Oncology
Morehouse School of Medicine
Degree: Master of Science in Clinical Research
MEDICAL SCHOOL
Peking University School of Medicine
Degree: Doctor of Medicine
POSTGRADUATE
Thomas Jefferson University, Kimmel Cancer Center
University of Miami Miller School of Medicine
Research Interests
Molecular pathways and therapeutic targets in brain tumors and cancer stem cells
The Liu lab mainly focuses on researching cell signaling pathways associated with
brain cancer, especially cell differentiation and tumor progression. The current standard
of care of surgery and radiochemotherapy for glioblastomas (GBM) is lacking and does
not result in improved prognosis. Accumulating evidence shows that the failure of
using current chemo- and radio-therapies to treat GBM and the resultant of high tumor
recurrence are attributed to the presence of a small subpopulation of glioma stem
cells (GSC), which is characterized by their stem cell-like properties and aggressive
behavior.
Our ongoing research projects include: 1) TRPM7 induces tumorigenesis and stemness through Notch and Stat3 activation in glioma.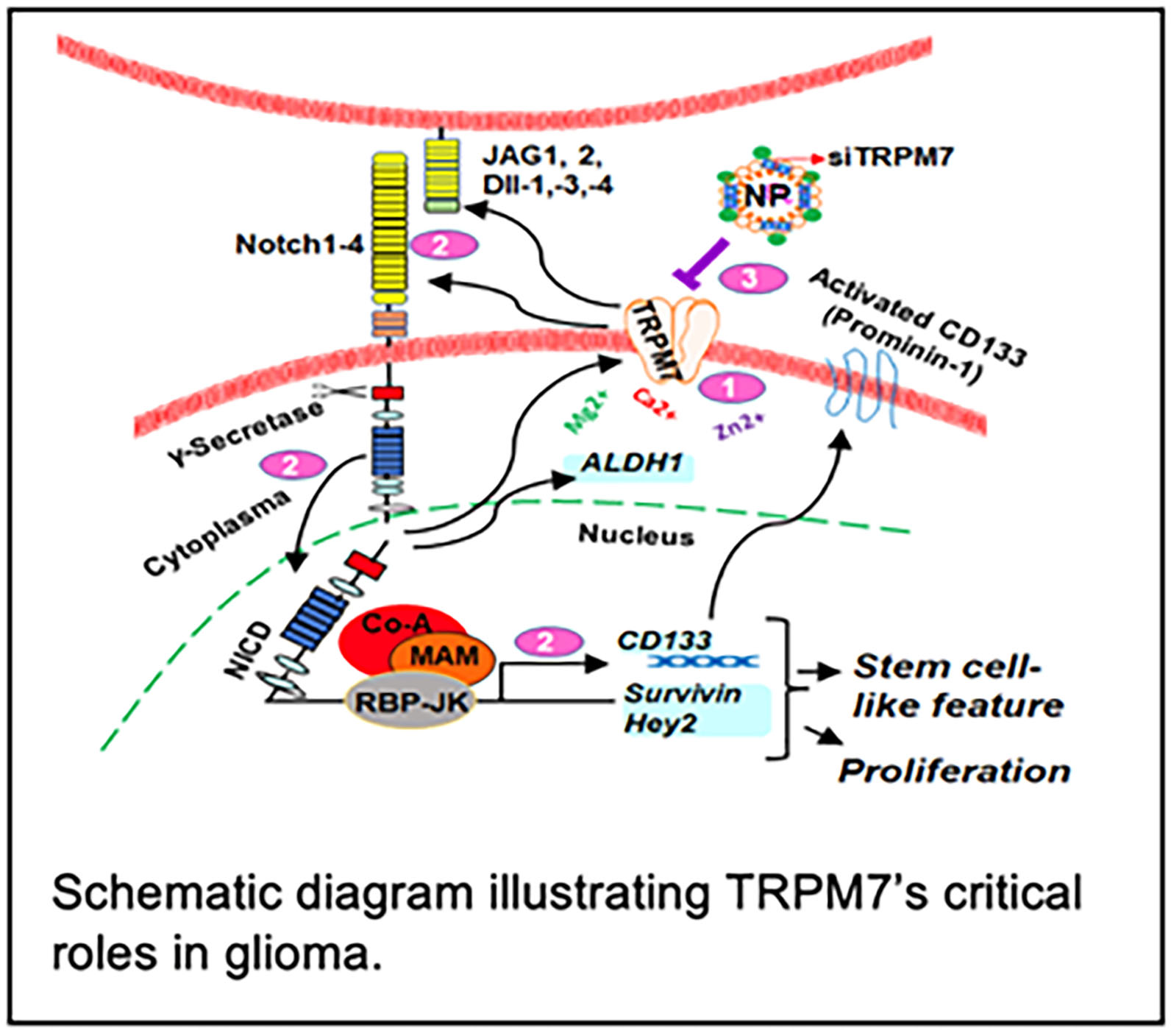
The proposed study will delineate the molecular mechanisms of TRPM7 in the development
and progression of glioma tumorigenesis and stemness as well as develop TRPM7 as a
novel drug target for glioma patients (see schematic figure). 2) Determination of racial disparities in glioma through Rap1b/Notch -induced tumorigenesis
and stemness. We are the first to report that the suppression of Rap1b inhibits proliferation,
migration, and invasion of malignant human gliomas, indicating that Rap1b may represent
a novel and promising target for therapeutic intervention of malignant glioma. The
proposed study will clarify the predictive role of Rap1b in GBM in patients, reveal
biological diversity across races and unaddressed health disparities, and evaluate
Rap1b’s potential as a therapeutic target. 3) Regulation of malignant glioma stem cells through acid sensor ASIC1. The available
mRNA microarray data from both Rembrandt and TCGA reveal that glioma patients with
high ASIC1 expression have preferable survival compared to those with low ASIC1 expression,
meaning that ASIC1 is associated with improved survival rates of glioma patients and
indicate that preserved susceptibility to extracellular pH may impair tumor growth. This
study will determine that ASIC1 functions as a tumor suppressor in glioma stemness
and tumorigenesis while the stimulation of ASIC1 activity may inhibit GSC self-renewal
and temozolomide induced resistance, and the novel knowledge from this study will
potentially be used to significantly impact the outcome of glioma patients' treatments
and contribute to ion transport proteins studies in other cancers. 4) Regulation of self-renewal and differentiation of neural stem cells by ASIC1a signaling.
Our overall hypothesis is that ASIC1a is functionally connected to neural differentiation
of neural stem cells (NSC) through the Notch signaling pathway. In this project, we
will determine ASIC1a’s regulation of NSC’s neural differentiation using hiPSC-NSCs
and ASIC1a depleted cells from ASIC1a knockout mice (ASIC1a-/-).
The overall goal is to better understand and develop mechanism-driven targeted therapies
for brain cancer patients. These include identification and characterization of genetic
and epigenetic changes during the initiation and progression of brain tumor, functional
characterization of these genes and/or proteins in cancer initiation, and development
of targeted therapies.
The Liu lab currently has positions open for postdoctoral candidates and interested
students learners for short- or long-term research experiences. Please contact Dr.
Liu at mliu@msm.edu for more information.
Current Personnel
- Vanajothi Ramar, PhD, Instructor
- Rajveer Singh Sidhu, PhD, Postdoctoral Fellow
Publications
- Ramar V, Sidhu RS, Pako O, Cisse CR, Guo AA, Li J, Stapleton K, Guo S, Wang G, Liu M. TRIM21 functions as an oncogene in glioblastoma by transactivating FOSL1 and promoting the ubiquitination of p27. Cell Commun Signal. 2025 Jul 1;23(1):313. doi: 10.1186/s12964-025-02325-6. PubMed PMID: 40598282; PubMed Central PMCID: PMC12211012.
- Guo S, Sidhu R, Ramar V, Guo AA, Wang G, Liu M. RNA Sequencing Identifies Novel Signaling Pathways and Potential Drug Target Genes Induced by FOSL1 in Glioma Progression and Stemness. 2025;19:157-176. doi: 10.2147/BTT.S509774. eCollection 2025. PubMed PMID: 40206361; PubMed Central PMCID: PMC11980931.
- Ramar V, Guo S, Hudson B, Khedri A, Guo AA, Li J, Liu M. Interaction of NF-κB and FOSL1 drives glioma stemness. Cell Mol Life Sci. 2024 Jun 10;81(1):255. doi: 10.1007/s00018-024-05293-1. PubMed PMID: 38856747; PubMed Central PMCID: PMC11335291.
- Guo S, Ramar V, Guo AA, Saafir T, Akpobiyeri H, Hudson B, Li J, Liu M. TRPM7 transactivates the FOSL1 gene through STAT3 and enhances glioma stemness. Cell Mol Life Sci. 2023 Aug 29;80(9):270. doi: 10.1007/s00018-023-04921-6. PubMed PMID: 37642779; PubMed Central PMCID: PMC10465393.
- Guo S, King P, Liang E, Guo AA, Liu M. LncRNA HOTAIR sponges miR-301a-3p to promote glioblastoma proliferation and invasion through upregulating FOSL1. Cell Signal. 2022 Mar 12;94:110306. doi: 10.1016/j.cellsig.2022.110306. [Epub ahead of print] PubMed PMID: 35292358; NIHMSID:NIHMS1790767.
Click here to view additional publications.
Honors and Awards
- 2009 Patent, Technology Transfer, ID: UMG-105, A Novel GREB1 Monoclonal Antibody, University of Miami
- 2016-2017 Minority-Serving Institution Faculty Scholar Award, American Association of Cancer Research
- 2016 Minority-Serving Institution Faculty Scholar Award, American Association of Cancer Research
- 2016 Health Disparity Research Training Program Award, The University of Alabama at Birmingham
- 2016 Pittsburgh Intensive Training in Hematology Research Scholarship, University of Pittsburgh
- 2016 Faculty Development Program, Morehouse School of Medicine
- 2017-2019 Scholar of the Clinical Research Education and Career Development, Morehouse School of Medicine
- 2018 Scholar of Frontiers in Stem Cells in Cancer, NIH/University of Pittsburgh
- 2019-2020 TxTM Pilot Grant Award, Morehouse School of Medicine
- 2021 NIH/Ad hoc reviewer, Clinical Neuroimmunology and Brain Tumors Study Section
- 2021 NIH/Ad hoc reviewer, Glioma, Multiple Sclerosis, and Neuroinflammation Study Section
- 2021 NIH/Ad hoc reviewer, Cancer Therapeutics and Drug Development Study Section
- 2023 NIH/Ad hoc reviewer, SuRE Program (R16), Special Emphasis Panel
- 2023 NIH/Ad hoc reviewer, ZTR1 RD-08 Understudied Proteins Associated with Rare Disease (R03)
- 2023-2025 NIH/Ad hoc reviewer, SuRE/SuRE First Program (R16), Study Sections (on 3 separate occasions)
- 2025 NIH/Ad hoc reviewer, SBIR/STTR Study Section
